The reactivated AETHER
Ide írhatod maximum 250 karakter hosszúságban a honlap leírását ill. szlogenjét. A leírás fontos a weboldal látogatottá tételében, ezért érdemes jól megszövegezni.
The crystalline structure of the atomic core
or
the Sindely atomic model
The science of our time is aware of how many protons and how many neutrons make up the nucleus of a given element. However, they have no idea what the geometric arrangement of nuclei is. In the absence of a better one, they think of a possible, random arrangement. It is assumed that the protons and neutrons form some quasi-evenly arrangement. But this layout is not like that, and we’ll see that it can’t be like that.
I can make some arguments in support of this view. In a random arrangement, the nucleons would have different energy minimums, so each atom of the same isotope would be slightly different from the others. That would not be clear either, why most nuclei are prone to decay and move from one random arrangement to another. Furthermore, the naive assumption that neutral neutrons mixed between repulsive protons will keep a sufficient distance between them is not valid either. For this, the neutron/proton ratio should be at least around 5. Neutrons would not shade the repulsive forces anyway, but would only slightly reduce them. In light of all this, the current random nucleus hypothesis does not seem viable to me.
 Figure 1. 46Pd106 Random model Figure 2. 46Pd106 Shingles model in the main section
Figure 1. 46Pd106 Random model Figure 2. 46Pd106 Shingles model in the main section
According to Sindely's model, protons are outside, while neutrons are exclusively inside. This is illustrated by the example of a medium-sized atom, 46Pd106 palladium. This sheds light on the meaning of the neutron surplus in the current parlance, as the inside of a spherical shell is always filled with something by nature. In our case with neutrons, the so-called. with a neutron excess. The inner neutron nucleus in the center of the neutron sphere may contain 0-54 neutrons. It is never spherical, too small for this, although it shows symmetry. For smaller license plates, the outer shells may not appear yet, only this inner core exists.
The outer neutron shell is bounded by quasi-spherical planes of angles 3-6. The number of an atom is determined by the number of neutrons in the outer spherical shell since one proton adheres to each outer neutron. Protons stand on the neutron nucleus like hedgehog spines and protect neutrons from spontaneous decay.
Here are some additional additions to the new core model system, without claiming completeness. The researcher assembled the geometric shapes from steel balls, partly using a magnetic table and then gluing them, as well as neodymium magnetic balls used too. He used about 100,000 bullets for modeling and made models of about 1,000 nuclei. *
During compilation, the imperfection of some shapes and shells, the formal problems of the model, was perceptible. The most common problems occurred outside the range of stable nuclei and in the case of odd neutron numbers.
If the inner core is too small, the shell will crease, as exemplified by the 30Zn63 zinc atom. It has 3 neutrons inside, which does not support the outer spherical shell enough. Therefore, a proton will push in there, pushing another neutron in front of it. The invading proton also converts to a neutron, so 5 neutrons form the inner core, and around it will be 29 neutrons, to which 29 protons adhere. This is a beta + decomposition, to a stable copper atom 29Cu63 from the unstable zinc atom creates.
If the inner core is too large, the shell of the model will become cracked and gaped. In the case of the 30Zn70 zinc atom, this is an excess of 10 neutrons and will be converted to a 31Ga71 gallium atom by decay. In this process, 2 neutrons are pushed radially to the surface of the nucleus. One moving out neutron enters the neutron shell and the other is pushed to the proton shell and is converted to a proton there. The mass number of the nucleus thus remains unchanged, but instead of 30 protons on the surface, it will now be 31, resulting in a 31Ga71 gallium atom.
Of particular interest is the inner neutron nucleus of the Uranium-235 atom. In the innermost core, neutrons form a 3x3x3 cube-shaped shape with 4-4 neutrons adhering to the sides. This set is only able to split at 1/3 and 2/3 along planes, as well as splitting the outer neutron shells and proton shells. This provided the answer to the fission mystery of the uranium atom, according to which U-235 splits into atoms of approximately 1/3 and 2/3 mass. The inner core of Uranium-238 has a completely different geometry, more stable. Therefore, it is not prone to splitting.
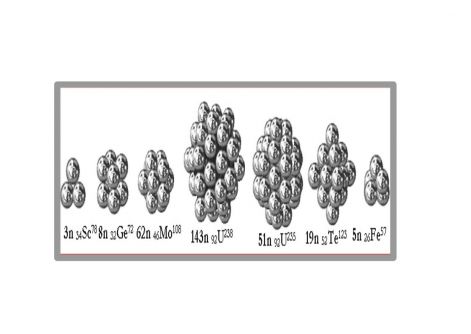 Figure 3. Internal neutron nuclei with example names
Figure 3. Internal neutron nuclei with example names
The impeccable internal logic of the large number of models prepared by the researcher, as well as its perfect harmony with the measurement results of isotope research, fully support the novel theory. However, I would add a deeper analysis.
Why is the Sindely nuclear model more stable than the one currently in use? In my opinion, better because the new theory has less repulsive and tensile force. Consider that the proton selected on the neutron nucleus of palladium is surrounded by 5 additional protons, but their electrostatic forces work against each other rather than repelling them. The effect of the additional 40 protons farther away is less because in proportion to the square of the distance. At the same time, the magnetic attraction of the proton to the protons below it is 2 orders of magnitude higher, since the quark circulating in the proton from huge circular currents of 10,000 amps. These create a very intense magnetic field. **
The above idea is confirmed by the behavior of the helium nucleus. This core is located in a deep energy pit so that the deuterium nuclei that fall into it radiate a lot of energy. The stability of this core, popularly known as the alpha particle, is also legendary. The balls are high-strength active magnets assembled in a row, in the p-n-n-p order. The outer protons protect the inner neutrons from decay, as they do with all other nuclei. The Helium atom best probably supports my theory. Not the so-called strong interaction, but the strong magnetic attraction holds the nucleons together in the nucleus.

Figure 4. The nucleus of helium
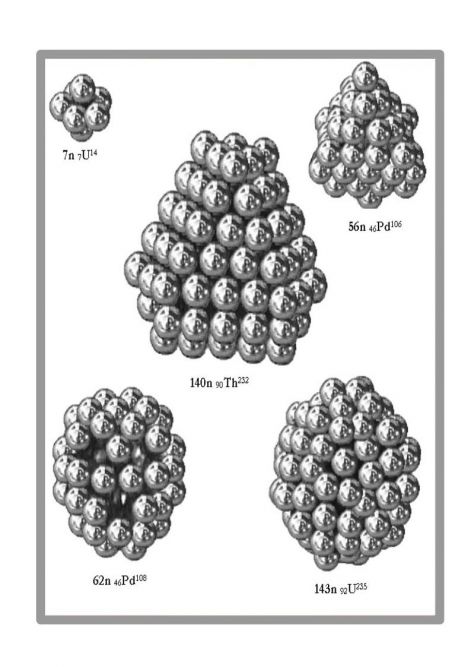
Figure 5 Nuclear nuclei depicted without protons
Date: 2019.Január hó
Tom Tushey
Mechanical engineer
Hobby physicist
Scientific writer
Relativity-expert
reactivated-aether@c2.hu
Honlapkészítés ingyen:
Ez a weblapszerkesztő alkalmas
ingyen weboldal,
ingyen honlap készítés...
Mai: 29
Tegnapi: 41
Heti: 29
Havi: 70
Össz.: 20 507
Látogatottság növelés
The reactivated AETHER - © 2008 - 2026 - reactivated-aether.hupont.hu